Abstract
INTRODUCTION: Severe injury provokes coagulopathy and systemic inflammatory response syndrome (SIRS) that, in concurrence, greatly increase the risk of life-threatening complications including bleeding, thrombosis, and multi-organ dysfunction syndrome (MODS), making it a leading cause of death and disability worldwide. For centuries, surgeons have observed hyperactivation of the enzyme plasmin (hyperfibrinolysis) following severe injury and its detrimental effects on bleeding in these patients. Recently, it has been demonstrated that the function of plasmin extends well beyond fibrinolysis to the modulation of inflammation and coagulation, suggesting it may alter the systemic inflammatory and coagulation response to severe injury. We hypothesize that early hyperactivation of plasmin augments systemic inflammation and coagulopathy following severe injury.
METHODS: To test this hypothesis, we used burn as a model of severe injury in which patients develop SIRS and coagulopathy without bleeding, allowing us to study the consequences of plasmin activation aside from hemorrhage. Thirty-one burn patients with >5% total body surface area (TBSA) burns admitted to the Vanderbilt University Medical Center Burn Unit were enrolled upon patient consent along with 10 healthy volunteers. Blood was collected serially for the length of hospital stay beginning upon admission. To study the clinical condition and control study variables, we administered a 30% TBSA burn to mice in a parallel study, collecting blood serially beginning at 1-hour post-burn (N=6/group). Control mice received similar preparation in the absence of a burn. Outputs assessed for humans and mice were the same. Plasma plasmin-antiplasmin complexes (PAPc) were used as a measure for plasmin activation, plasma IL-6, IL-1β, and TNFα were used to measure systemic inflammation, and plasma antithrombin III (ATIII) deficiency and platelet count were used to measure coagulopathy. To study the role of plasmin(ogen) in burn-induced coagulopathy and inflammation in a preliminary experiment, plasminogen+/- mice with 50% normal circulating plasminogen and plasmin activity received a similar burn. Both human and animal studies were previously approved by Vanderbilt University IRB and IACUC respectively.
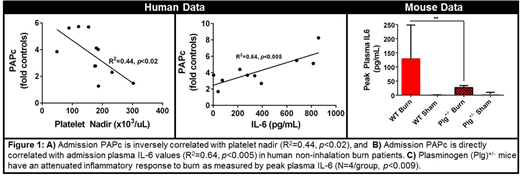
RESULTS: In burn patients without inhalation injury, PAPc levels significantly increased in proportion to the severity of injury measured by %TBSA (p<0.03). Additionally admission PAPc levels were inversely correlated with platelet nadir (R2=0.44, p<0.02) and directly correlated with peak IL-6 levels (R2=0.64, p<0.005) (Figure 1A-B). Patients with inhalation injuries did not exhibit significant correlations for these measurements. While ATIII deficiency was only detected in 4 out of 31 burn patients, burned mice exhibited a significant ATIII deficiency between 1 and 6 hours post-burn (p<0.01) compared with controls. In parallel with the human results, mice with burns developed a significant platelet deficiency and dramatically increased plasma PAPc and IL-6 levels at 24 hours post-burn(p<0.001). Early studies in Plg+/- mice demonstrated a markedly reduced peak plasma IL-6 levels post-burn (p<0.009) (Figure 1C). Changes in plasma IL-1β and TNFα were weakly sensitive to injury severity.
CONCLUSION: Studies in severe injuries and elective surgeries have demonstrated that early inhibition of plasmin activation by antifibrinolytic drugs not only reduced bleeding, but also systemic inflammation. Our results suggest an association between plasmin activation, platelet loss, and systemic inflammation following burn injury in both human and mouse models. While the study in Plg+/- mice implicates plasmin in burn-induced inflammation, future studies will examine the mechanistic role of plasmin activation in burn-induced systemic inflammation and coagulopathy using genetic and pharmacologic tools to manipulate plasmin in a mouse model. While the systemic response to burn injury is similar to that of other severe injuries, future studies will investigate the relationship between plasmin activation, coagulopathy, and SIRS in general trauma. As a critical modulator of hemostasis and inflammation, our findings support plasmin as a potential therapeutic target for regulating trauma-induced coagulopathy and SIRS, beyond inhibition of fibrinolysis.
Schoenecker:OrthoPediatrics: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal